INDUSTRY UPDATES
May 17, 2022
New Study Demonstrates CBD’S Strong Safety Profile, Amplifies Calls For FDA Regulation
FOR IMMEDIATE RELEASE
March 17, 2022
NEW STUDY DEMONSTRATES CBD’S STRONG SAFETY PROFILE, AMPLIFIES CALLS FOR FDA REGULATION
Observational Data Collected On Long-term Consumption of Oral CBD Shows No Correlation To Safety Issues Raised by FDA
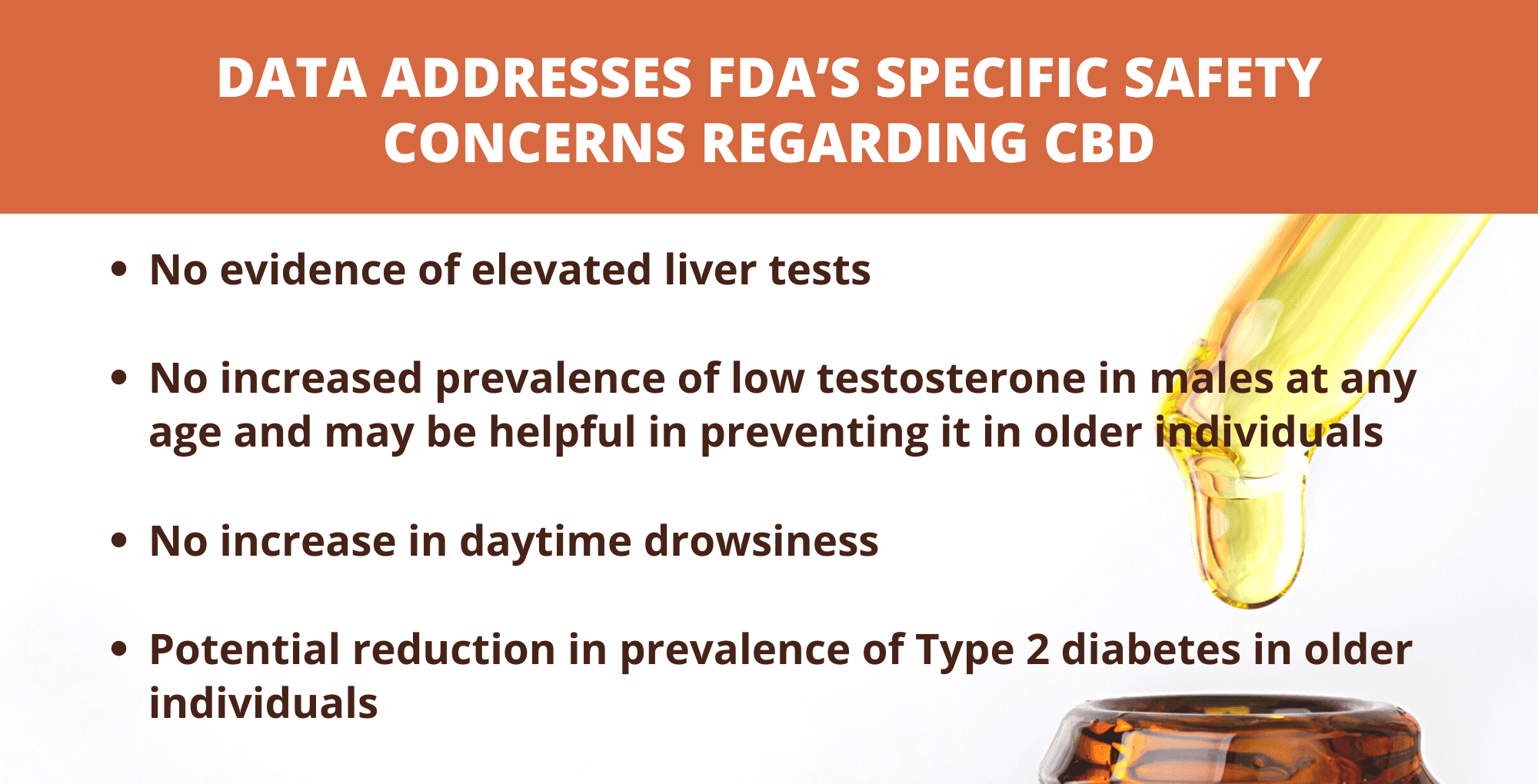
A recently released, comprehensive safety study confirms prior research that orally-ingested cannabidiol (CBD) has a strong safety profile, providing data that addresses FDA’s specific safety concerns regarding CBD, with the results indicating that daily consumption across a range of typical retail products and serving sizes is not associated with elevated liver tests, low testosterone levels, or daytime drowsiness. The results renew calls from the hemp and CBD industries urging FDA to take steps to regulate CBD now, and calling on Congress to act if FDA continues to delay.
The study was completed on March 22, 2022, and involved 1,061 participants, providing a 98% power rating on its results, which is statistically significant. Validcare contracted with 17 CBD companies and acted as the Contract Research Organization, which included gaining feedback from FDA on the protocol, conducting the study and publishing its results. The study abstract is available here.
The first cohort of the study, which was peer-reviewed and published in Cannabis and Cannabinoid Medicine last year, observed 839 individuals taking CBD produced by twelve companies with the primary end point being liver function. The second cohort included an additional 222 individuals taking CBD produced by an additional five companies. Their participation strengthened the statistical reliability of both the liver safety results and achieved statistical relevance for both sleep and testosterone results. The methods for both phases were the same. Adults 18-75 years of age across the United States, taking CBD orally for a minimum of 30 days, were recruited by seventeen individual CBD product companies in this decentralized, observational study and provided their standard CBD regimen.
All product companies supplied a third-party certificate of analysis (COA) which was confirmed by a neutral third party to ensure composition of the supplied product matched both the label and the supplied COA. The companies that funded and provided product for the study include Asterra Labs, Cannacraft, CBDistillery, CBD American Shaman, Charlotte’s Web, Columbia Care, Garden of Life, Global Widget, HempFusion, Impact Naturals, Kannaway, Kazmira, Medterra CBD, SunMed CBD, and Tauriga Sciences.
“The data in this study looks really good; it’s highly significant, and the chances of it being wrong are very, very small,” stated Dr. Robert Kaufmann, Director of Research for Validcare and former Professor of Medicine at Southern Illinois University School of Medicine. “I am very hopeful that this data will allow the FDA to regulate these popular CBD products.”
It has been over four years since the 2018 Farm Bill legalized hemp and non-intoxicating hemp derivatives like CBD. Within hours of the Farm Bill’s signing, then-FDA Commissioner Scott Gottlieb recognized the “clear interest of Congress in fostering the development of appropriate hemp products” and acknowledged that the FDA “has the authority to issue a regulation,” which would allow for the lawful marketing of CBD as a dietary supplement. In March 2020, the FDA released a Congressional report and public statement on potential regulatory pathways for the sale of hemp-derived CBD products, listing liver injury as the top concern for consumer safety, along with “male reproductive toxicity, or damage to fertility in males or male offspring of women.” The agency committed to working efficiently to further clarify a regulatory approach to these products, “using science as our guide and upholding our rigorous public health standards.” Still, the FDA has not taken any concrete steps to regulate CBD, claiming that it needs more real-world data to move forward.
“We are excited to report that the ‘real-world data’ that FDA has been soliciting addresses the agency’s safety concerns,” stated Jonathan Miller, General Counsel to the U.S. Hemp Roundtable, the hemp industry’s national advocacy organization. “The time has come for FDA to regulate CBD and other hemp derivatives. If FDA does not act, we call on Congress to pass legislation such as HR 841, HR 6134 and S. 1698 which would require the FDA to develop regulatory pathways for the sale of hemp extracts like CBD in ingestible form.”
“As an advocate for ‘the science behind the supplements,’ CRN is particularly encouraged by the rigor and thoroughness of this new research on the safety of CBD. We applaud the companies who supported this new examination in a real world setting for expanding the understanding of CBD. We are hopeful that this study will help open a legal pathway for using CBD in dietary supplements,” stated Steve Mister, President & CEO of the Council for Responsible Nutrition.
“This study reaffirms the safety of Charlotte’s Web hemp-derived CBD extracts and are proof of the hemp CBD industry working cooperatively to support rigorous scientific research to inform regulators, the FDA, and the U.S. Congress,” said Charlotte’s Web Sr. Vice President and the Company’s President of its CW Labs Division Tim Orr. “With 1,060 participants in two cohort studies, industry stepped up and delivered the requested science for hemp CBD, again, by providing evidence outcomes in liver safety, drowsiness, testosterone impact and diabetes prevalence.”
“We are proud to have participated in this ground-breaking study on CBD products and feel verified in our products excellent results in the testing. These results bode very well for supporting the hemp industry against the fears that FDA had previously stated which had been a hurdle in their regulatory process,” said Vince Sanders, Owner CBD American Shaman. “We are excited they can now rely on this study in verifying CBD products as safe for the human liver, have no effects of day-time drowsiness, and no negative effect on low testosterone levels or reproductive harm of male participants or the male offspring of women in the study.”
“In over two million customers, Medterra has first hand seen the safety and benefits of CBD. With this landmark safety study completed, we believe the FDA now has clear confirmation on CBD’s safety and will hopefully provide prompt guidance for CBD as a dietary supplement,” stated Gregory Reeder, Chief Executive Officer and International Managing Director of Medterra.
“Participating in this study has allowed us to help provide regulators, scientists, product formulators, and other stakeholders with the evidence needed to prove the safety profile of CBD,” said Blake Schroeder, CEO of Medical Marijuana Inc, and its subsidiary, Kannaway. “We hope that this, in addition to our other research efforts in Brazil and Mexico, will not only help break the stigma around CBD but that it will also help legislators understand the importance of free, legal access to the entire cannabis plant.”
“The study trial is exceptional as it validates the safety of leading CBD products and brands as tested across a wide variety of people, including at high dosages,” says Vassili Kotlov, a founder and CEO of Impact Naturals. “Our company was founded on scientific rigor, and participating in this landmark study was a top priority. We are passionate about the science of cannabinoids, and validating CBD safety is the foundation for our innovative products.”
“We’re glad to have served as a sponsor of this study and are very pleased with the outcome. At Asterra Labs, we remain committed to the research, development and manufacturing of quality hemp based products with a keen consumer-directed focus on identity, purity and potency,” said Kevin Sills, CEO of Asterra Labs.
“One of the tragedies of federal cannabis prohibition has been the restrictions on the research into the safety and efficacy of cannabinoid-based therapeutics. We’re so appreciative to join other industry leading brands pioneering critical research into the safety profile of CBD, one of many potentially beneficial cannabinoids found in hemp and cannabis. This is a significant step for patients and consumers,” stated Tiffany Devitt, CannaCraft, Inc. Chief of Regulatory Affairs.
###
